Semen Analysis Testing
A Comprehensive guide
Semen Analysis Testing- An Introduction
Evaluating the fertility potential of a man generally begins with semen analysis testing. The semen analysis is not a perfect test, but is currently the best predictor that we have available for determining a man's general fertility potential. This section will explain the proper way of planning and collecting a semen specimen as well as review how to accurately interpret the results of the test. Some potentially reversible semen analysis-related problems (such as antisperm antibodies) are also discussed.
Semen Analysis Testing- When, Where, Why, and How
The semen analysis is the single most effective predictor of male fertility potential that is currently available. The American Society of Reproductive Medicine recommends that all men should have semen analysis testing done at the same time as initiating testing from the female side in couples having trouble conceiving a child.
We know that the semen analysis is not a perfect test. Some men with low sperm counts and poor sperm quality can achieve pregnancies without assistance, while others who have completely normal semen parameters are unable to establish a pregnancy with their partner. In general, though, men with better semen parameters have an easier time initiating a pregnancy (both naturally and with the assistance of female fertility treatments), and so it’s beneficial to try and optimize these parameters as much as possible.
WHEN TO GET A MALE FERTILITY EVALUATION
If you are trying to have a child and have found your way to this website, then you or your partner probably have good reason to have a male fertility evaluation if one has not been performed already. The “official” guidelines are that the male should have fertility testing if a couple has been unsuccessful at establishing and maintaining a pregnancy after one year of trying. There are many exceptions to this general rule, of course. If the man has a known history of risk factors that can impact his fertility (for example, previous scrotal surgery, chemotherapy, or genetic problems), then earlier testing would be a good idea. Also, if the woman is thirty-five years of age or older, then checking the man’s fertility potential after six months of trying (instead of twelve) would not be unreasonable. Some couples just choose to check semen testing earlier in the fertility process, or want to evaluate the man’s fertility potential before they even start trying for a child. There is no problem with these approaches if it makes the couple feel more comfortable with the process.
HOW MANY SEMEN ANALYSES SHOULD BE PERFORMED?
Normal Semen Parameters
The standard recommendation is a single semen analysis is adequate if all of the parameters are in the normal range. However, a normal semen analysis does not mean that a man is not sub-fertile, so there a few caveats to consider:
#1) Semen analysis testing only gives us a rough indication of a man's true fertility capabilities, and may miss other otherwise hidden biochemical and/or genetic abnormalities that can negatively impact a man's fertility potential. Therefore, even if a man has normal semen analysis testing, he may benefit from some basic lifestyle-related interventions that can increase his chances of successfully establishing a healthy pregnancy. These interventions are reviewed in the "Sperm Boot Camp" section of this website.
#2) If a man has normal semen analysis testing but the couple is still not able to establish a pregnancy after a subsequent reasonable period of time trying (e.g. 6 to 12 months), then repeat semen analysis testing (and possibly other tests, such as hormonal and/or sperm DNA fragmentation testing) may be indicated.
Abnormal Semen Parameters
When abnormalities are present on semen analysis testing, then at least two semen analyses should be performed, at least ten weeks apart. If the first analysis is abnormal, then the next step can be to see a male fertility specialist, after which the second semen analysis can be performed after some basic testing and interventions have been undertaken.
Why a Ten-Week Interval Between Semen Analyses?
Ten weeks is the length of a spermatogenic cycle, which is the time that it takes for a spermatic precursor cell to become a fully mature sperm. Any activities that can have a negative impact on sperm quality (such as sitting in a hot tub, smoking, etc.) can damage all of the sperm in the body at that point in time. It takes approximately ten weeks to get the damaged batch of sperm completely out of the body and replace it with a new, healthier set of sperm. Therefore, to get a more accurate picture of a man’s fertility potential, it is best to sample semen parameters from two separate batches of sperm if abnormalities are found on the initial testing. Also, any interventions that are performed to improve semen parameters generally take at least 10 weeks to be fully reflected in subsequent semen analysis testing.
WHERE TO GET SEMEN TESTING
The accuracy of a semen analysis depends on the skill and training of the laboratory technicians who perform it. I have seen semen analysis results from more than a hundred labs over the years, and the variability in quality is striking. Therefore, it makes sense to find a lab in your area that can offer a quality semen analysis, which is going to provide you with the most accurate information possible.
There are two main types of labs that you can choose to perform your semen analysis: hospital-based labs and fertility-specific labs. Hospital-based labs carry out a wide range of general medical testing. I would also include in this category large regional or national laboratories (such as Quest Diagnostics) that sometimes offer semen testing. While these general labs are good at other fertility-related testing such as blood hormone levels, I have found the quality of their semen analyses to be highly variable.
Accurate semen analysis requires highly trained technicians to perform specialized testing, including determining sperm density (numbers of sperm present), motility (how well they are swimming), and morphology (whether they have normal shapes). Accuracy depends greatly on how the specimen is handled and processed as well as on the skill of the technician making the measurements.
Fertility-specific labs are devoted only to fertility-related testing and treatments. These labs are associated with an IVF group, and therefore their staff are quite knowledgeable and experienced. The semen analyses they perform usually utilize the latest diagnostic criteria, and the results are generally more consistent and accurate than those from hospital-based labs.
In addition, most fertility-specific labs are set up to have private collection rooms with locking doors (something not generally available in hospital-based labs). Collecting the specimen at the lab eliminates travel time and transport issues, which can affect the outcome. Some fertility-specific labs do allow home collections, but under very strict timing and transport guidelines.
HOW TO FIND A FERTILITY-SPECIFIC LAB
You can Google local IVF doctors and then call to find out whether they are associated with a fertility-specific lab. You can also go to the website of the Society for Assisted Reproductive Technology (www.sart.com) and click on “IVF Success Rates” which has a drop-down tab where you can select "Find a Clinic". You can then search by Zip Code, State, or Region to find a list of the IVF programs located near you. You can then choose a local lab and get its contact information.
Once you have located a local IVF group, call the clinic and ask to be transferred to the lab, which can schedule you for a semen analysis.
What If There Is No Fertility-Specific Lab Nearby?
All large cities (and most medium-sized ones as well) will have an IVF lab nearby. However, for couples living farther from an urban center, a hospital-based lab may be their only choice. But such couples should keep in mind that if the results of the analysis do not seem to match up with their clinical situation, then they might want to try another nearby lab or make a road trip to the nearest large city to get more accurate testing.
ReproSource @Home Collection Kit
Another potential option for couples without a local fertility-specific lab is the ReproSource @Home Collection Kit. This is a home collection kit that is shipped directly to the patient. The patient collects his specimen and then sends if back by overnight shipping back to ReproSource which then performs standard semen analysis testing. Through certain technological innovations in specimen stabilization, extremely accurate results are able to be obtained which closely match those from a fertility-specific lab. The cost is generally a little higher for the ReproSource testing due to the extra shipping costs, but this is an good option for couples who do not have access to a nearby quality semen analysis lab. It is also an option for men who are able to collect a specimen in the comfort of their own home, but are unable to effectively collect a specimen in the artificial environment of the semen analysis lab (and live too far from the lab to be able to get a home-collected specimen there within the necessary 30 minute time window for accurate results testing).
OTHER OPTIONAL Tests that Can be performed during a semen analysis
A standard semen analysis generally checks the ejaculate volume, sperm density, motility, and morphology (though in some labs the morphology is an optional add-on). Many labs will also generally check a semen pH (and sometimes fructose) and look for the presence of elevated numbers of white blood cells. Optional tests can also be added on to the standard semen parameters when the clinical situation dictates. These include:
1) Post-Ejaculatory Urinalysis
The post-ejaculatory urinalysis (PEU) involves the man providing a urine specimen collected in a separate collection cup after ejaculation. PEU testing looks for the presence of retrograde ejaculation and is generally checked in the presence of persistently low ejaculate volumes (<1.5cc). The Ejaculatory Dysfunction section of this website reviews retrograde ejaculation in more detail.
2) Immunobead Testing
This measures for the presence of antisperm antibodies, which is often accompanied by clumping (called "agglutination") of sperm on the semen analysis. See the Antisperm Antibody section of this website for more detailed information.
3) Sperm Washing
A test sperm wash is used to prep the sperm like it would prior to an intrauterine insemination (IUI). A test wash calculates a post-wash total motile sperm count which can be used as an indicator of the relative success rates of any subsequent IUI cycles. See Female Fertility Treatments for more information on IUIs.
4) Sperm Cryopreservation
Sperm can be frozen and stored by the lab. There are several potential reasons for sperm freezing and storage. Some men who are diagnosed with cancer choose to bank sperm if they are planning on undergoing treatments (such as chemotherapy and/or radiation) which have the potential to permanently impair sperm production. Men with very low sperm counts can freeze sperm as back-up in anticipation of an upcoming IVF cycle, in case they have no usable sperm on the day of their wife/partner's egg retrieval. Men with known ejaculatory and/or erection problems also sometimes bank sperm in case they are not able to produce a specimen on the day of their wife/partner's female fertility treatment. In addition, some soldiers who are getting ready to be deployed freeze sperm for use in female fertility treatments that can be used by their wife/partner when they are gone.
THE COST OF SEMEN ANALYSIS Testing
The exact cost is going to vary between laboratories, but it generally falls in the $100 to $200 range. Depending on your state’s insurance coverage laws as well as the specifics of your insurance plan, the testing may or may not be covered. Sometimes a certain lab is covered by your insurance plan but another is not, so doing some research beforehand can potentially save you some money. However, if you have a fertility-specific lab in your area but find that the semen analysis is either cheaper or covered by insurance at a local hospital lab, I would still recommend getting the testing performed at the fertility-specific lab, since the results will likely be more accurate and useful.
See "Cost Guide" Section for more specific pricing information
PROPER COLLECTION OF THE Specimen for semen analysis testing
Collecting a semen specimen for analysis can be an embarrassing and stressful endeavor for some men. Here are some recommendations for making this process as smooth and accurate as possible. Always check with the fertility lab that you are working with for specific instructions, as they may vary somewhat from lab-to-lab.
Step #1: Schedule the appointment
Unlike blood testing labs, which often allow patients to simply drop in, fertility labs almost always require an appointment. Because semen analysis is generally performed manually by a technician, the lab needs to set aside the proper amount of time. You usually need an order signed by a licensed medical care provider to get a semen analysis performed.
A sample semen analysis form is available in the "Forms" section of this website
Step #2: Coordinate where the specimen is going to be collected
Each semen analysis lab is going to have its own recommendations on specimen collection. Many fertility-specific labs have designated collection rooms at their facilities, with locking doors for privacy. Other labs prefer specimen collection at home, but only if the specimen can be delivered to the lab within thirty minutes. If you are going to do a home collection, you still need to have scheduled an appointment time for specimen drop-off. You also need to stop by the lab beforehand to pick up the recommended collection cup (generally a wide-mouthed sterile container with a tight-fitting lid). After collecting the specimen, seal the container, tuck it into a pocket (to keep the specimen near body temperature), and take it straight to the lab.
Step #3: Determine the number of days of abstinence
It is generally recommended that you abstain from ejaculation for two to five days prior to semen analysis. The male reproductive system needs some time to replenish the semen supply between ejaculations, so if you abstain for less than two days, the sample can contain inadequate amounts of fluid and sperm. If you wait longer than five days, the sperm can get old and start to die off, so the sperm quality can appear inaccurately decreased. I tell my patients to aim for three or four days of abstinence prior to testing, so that if there are last-minute timing issues, there’s an extra day of cushion on either side.
TROUBLESHOOTING COLLECTION PROBLEMS During Semen analysis testing
Missing Part of Specimen During Collection
Trying to collect a semen specimen in a rather small cup is usually a new experience for most men, and it is quite common for men to miss collecting part of the specimen, especially the first time or two they try. I therefore recommend that men “practice” once or twice at home prior to the actual week of their semen analysis, using a similar type of wide-mouth cup (such as a Dixie cup). If you still miss part of the specimen on the day of testing, do not try to scoop up the missed part into the collection cup—you don’t want to introduce outside bacteria or foreign matter into the specimen. Instead, when you turn in the specimen cup, just let the lab know that you missed part of the collection, and an estimate of what percentage of the specimen you think you lost so that this can be reported and taken into consideration when evaluating the results.
Need for Lubrication to Collect Specimen
Many lubricants can damage sperm quality if they come into contact with the semen. However, masturbation without lubrication can be unpleasant (and sometime not possible) for some men. There are isotonic lubricants, such as Preseed, which do not seem to have a negative impact on sperm quality. If you think that you are going to have a problem with collecting a specimen without lubrication, then ask the lab if you can use an isotonic lubricant (which can be purchased without a prescription and is found at most drugstores).
See "Lubricants" section for more detailed information on this topic.
Inability to Collect a Specimen
Sometimes men are unable to collect a specimen at all, and there can be many reasons for this. Some men just cannot collect in a strange environment (such as a fertility lab’s collection room), while others have a mental block about collecting a specimen for fertility testing. Other reasons include religious beliefs that discourage masturbation or medical problems that impact a man’s ability to get an erection and/or ejaculate. Some common causes of sperm collection problems are reviewed below:
1) Known erectile dysfunction/ejaculatory problems
Some of these men have a long-standing history of erectile dysfunction or ejaculation problems, and for them the difficulties with specimen collection can be anticipated beforehand. For these types of issues, please see the sections “Erectile Dysfunction” and “Ejaculatory Problems”
2) Inability to Collect a Specimen in a Strange Environment
Most men find the prospect of providing a semen specimen for evaluation a stressful and embarrassing undertaking. Some men just cannot “perform” in a collection room at the lab, whether due to problems with achieving and/or maintaining an erection, or due to difficulty reaching climax.
If the problem is primarily one of achieving or maintaining an erection, sometimes taking an erection-enhancing medication prior to a planned collection may be helpful. For transient sperm collection difficulties in men who normally have fairly good erections, taking 100 mg of Viagra, 20 mg of Levitra or Staxyn, or 20 mg of Cialis one hour prior to the scheduled collection time, or 10 mg of Stendra fifteen to thirty minutes beforehand, may be of benefit. See “Erectile Dysfunction” section (link above) for details on the proper use of these medications, as well as information about side effects and contraindications to taking them.
If collection-related erection problems persist despite use of these medications, or if the primary problem is an inability to reach climax and orgasm in an unfamiliar environment, then collecting a specimen in the comfort of one’s home may be helpful. As noted earlier, home collections must be closely coordinated with the lab. Specimens must be kept warm (around body temperature, so keeping them tucked into a pocket is fine) and must make it to the lab within thirty minutes. Also, a proper sterile collection cup must be obtained from the lab prior to specimen collection. The ReproSource @Home Collection Kit is another option (described earlier in this section) for men who are only able to collect a specimen at home but who live more than 30 minutes away from the fertility lab.
3) Inability to Effectively Masturbate in Any Location to Collect a Specimen
Some men who have masturbated normally in the past just have a mental block when trying to collect a specimen for evaluation and cannot reach orgasm even in the comfort of their own home. In these situations, sometimes the man’s partner needs to get involved to help with collection of the specimen; however, be careful to follow the lab’s guidelines regarding allowed lubrication and no use of saliva. Coitus interruptus (having intercourse and then pulling out right before ejaculation) is not recommended: it can be difficult to collect the full specimen, and there is potential for picking up contaminants from the microorganisms normally found in the vagina. A better option is using a special collection condom, which can be then given to the lab technician. Regular condoms cannot be used; you must obtain a specially made collection condom beforehand. Ask the lab if it will provide one or if you will need to purchase one on your own. An example of a commercially available collection condom is the Male Factor Pak, which costs around $15 at Amazon.com. Always check with the lab before using a collection condom to make sure that this is okay with them.
4) Lack of Experience with Masturbation
Although most men begin masturbating with the onset of puberty, some men make it to adulthood without ever having masturbated. While sociological studies have shown that the vast majority of men (at least 95 percent) masturbate, certain religious groups officially discourage masturbation, and some men from these religious communities may be unwilling or unable to produce a semen specimen for analysis. Depending on the individual’s particular beliefs, a special collection condom (mentioned above) could potentially be used in these circumstances. Working with a local sex therapist can be an effective approach in some situations. Post-coital testing can also be used with some couples to see if decent numbers of motile sperm are reaching the cervical mucus, although exact semen parameters cannot be measured.
See "Uncommonly Used Sperm Testing" for more information on post-coital testing
Planning for IVF with a History of Transient Sperm Collection Problems
If a man has had collection difficulties in the past and his partner is scheduled for an upcoming IVF procedure, I strongly recommend that he collect and freeze sperm well prior to egg retrieval. When eggs are retrieved for IVF, they typically need to be fertilized within about six hours. This can place quite a lot of pressure on a man to “produce” during this six-hour window. If the couple has some backup frozen sperm in reserve, then the pressure is off. In the event that he cannot produce a specimen that day, the frozen sperm can be thawed and used for the IVF cycle, and in most cases the success rate is essentially unchanged. Most IVF labs now offer egg freezing as a last resort, but it is always preferable to not have to freeze unfertilized eggs if it is avoidable.
Accurate Interpretation of Semen Analysis Test Results
There exists quite a bit of controversy about what are considered “normal” semen parameters, as well as what “normal” means when it comes to male fertility. So let’s make a few general statements about semen analysis testing before we dive into the details.
1. Semen analysis testing is not a perfect predictor of male fertility potential, but it is the best overall general test that we have available at this point in time.
2. The best general predictor of overall sperm quality is the total motile count (TMC).
3. In general, the higher the numbers and the better the quality of sperm present, the greater the fertility potential of that man, so it makes sense to try to optimize semen parameters in all couples who are trying to conceive. There is evidence that this is even the case in couples who are undergoing treatments such as in vitro fertilization. When IVF/ICSI was first being developed, some researchers thought that this technology would compensate for any deficiencies in sperm quality. However, there is now mounting evidence that better-quality sperm have the potential to provide higher success rates when used with these types of female fertility treatments. Therefore we feel that all couples can potentially benefit from optimizing the man’s sperm quality.
DEFINING “NORMAL” SEMEN PARAMETERS on Semen Analysis Testing
You might think that three and a half decades of research on typical semen parameters would have resolved most of the questions and controversies about what “normal” semen parameters should be. Unfortunately, both patients and physicians are still routinely confused about what “normal” means when it comes to interpreting semen analysis results. I regularly see men in my office who have good semen parameters but were told that they have significant abnormalities. On the other hand, every year large numbers of men are told that their semen parameters are normal when in fact they have significantly decreased fertility potential. Why all the confusion?
Part of the problem lies in the fact that specific sperm counts and quality do not always match up nicely with fertility outcomes. Some couples with really low sperm counts are successful at establishing a pregnancy naturally, while others with completely normal semen parameters (and no problems found on female fertility testing) end up needing to utilize advanced treatments such as IVF/ICSI, and sometimes these are not even successful. Elements of chance as well as other, yet-to-be-identified fertility problems that do not show up on currently available fertility testing certainly play a role. But in general, the numbers and quality of sperm are the strongest predictor of a man’s overall fertility potential that we have at this time.
"NORMAL" SEMEN PARAMETERS on semen analysis Testing
The semen analysis test evaluates multiple characteristics, but the four that are generally considered to be the most important are:
1) Ejaculate volume (the amount of fluid that comes out with ejaculation)
2) Density (the number of sperm present)
3) Motility (the percentage of those sperm that are swimming)
4) Morphology (the percentage of sperm that have normal shapes)
The following table shows the most recent (2010) World Health Organization criteria for “normal” semen analysis values, as well as the 1999 WHO criteria for comparison:
At first glance this seems pretty clear-cut. If a man has a sperm count of at least 15 million sperm/cc, at least 40 percent of the sperm are swimming, and at least 4 percent of them have normal shapes, then his fertility potential is just fine and any fertility problems must be either just bad luck so far or problems from the female side, right? Not necessarily.
Let’s look at how the WHO came up with these criteria. The WHO evaluated 4,500 men from fourteen countries and based the “normal” values on a subset of 1,800 men who were able to conceive naturally within twelve months. Of these 1,800 men, the bottom 5 percent of semen parameter values were labeled “abnormal,” with the remaining 95 percent of values being labeled “normal.”
Consider a man with a sperm count of 15 million who was told that his sperm count was “normal.” How comfortable should that man feel about his fertility potential knowing that according to the WHO data, of the men who were able to successfully conceive within a year’s time, 95 percent had a higher sperm count than he has? In addition, of the men in the study who were not able to conceive naturally within a year, 85 percent had a sperm count higher than 15 million. Similar comparisons can be made for motility and morphology results as well.
Overall, an estimated 50 percent of infertile men (defined as unable to conceive naturally within a year) have semen parameters that fall within the “normal” range as defined by the WHO criteria. Obviously, the WHO’s “normal” ranges should undergo further scrutiny.
A POTENTIALLY BETTER DEFINITION OF "NORMAL" MALE FERTILITY POTENTIAL on semen analysis testing
I would argue that a better definition of male fertility potential would be to use the following parameters as “normal”:
Later sections in this website will explore in more detail each of the individual parameters of the semen analysis test, as well as explain why I consider the above criteria to be the best estimate of "normal" fertility at this point in time.
VARIATION of semen parameters on semen analysis Testing: WHEN NOT TO WORRY
Many patients are confused and frustrated when they see unexpected differences in their numbers between one semen analysis and another—especially when parameters that seemed normal on an earlier test appear to be going in the wrong direction on a later test. But such differences aren’t always something to worry about.
Scientific studies that examined ejaculations by the same man over a period of time show significant week-to-week variation among samples. This natural variability can make accurate determinations of relative fertility or responses to male fertility treatments challenging. In general, I consider about a 20 percent swing in semen parameters to be within the normal expected range of natural variation due to the body’s normal mildly fluctuating sperm production process. Larger swings are more likely to represent a real change in sperm quality for some reason.
When questions arise as to whether an observed change is due to natural variation, a real physiologic change, or lab error, additional semen analyses can be helpful in observing general trends over time. For example, if I have a patient who has two semen analyses with markedly different results, I will typically order a third “tie-breaker” analysis at least ten weeks after the second test to get a more accurate picture.
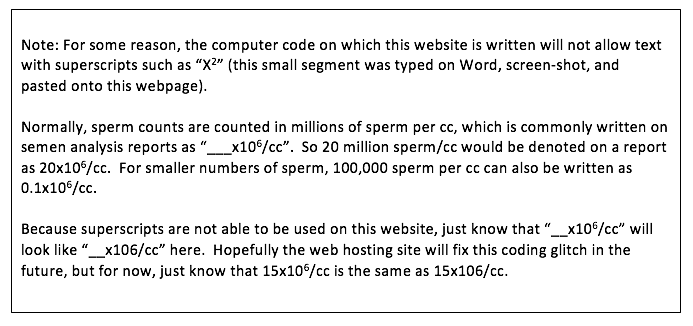
Sperm Density on Semen Analysis Testing
Sperm density is the number of sperm that are present per milliliter (cc) of semen. Sperm density is typically reported in units of millions of sperm per cc.
While the latest WHO criteria designate a normal sperm density as 15 × 106/cc or more, I think a sperm density of 40 × 106/cc or more may be more appropriate for a truly normal sperm density. However, using the total motile count (TMC) is arguably an even better way of judging male fertility potential.
A few commonly used terms regarding sperm density:
Oligospermia: any sperm density less than what is considered normal
Severe oligospermia: typically considered to be less than 5 million sperm/cc
Azoospermia: no sperm at all seen in the ejaculate
Virtual azoospermia: only a very small number of sperm (sometimes defined as less than
100,000 sperm/cc)
Of note, azoospermia and virtual azoospermia are explored in greater detail in separate sections of this website.
SPERM MOTILITY on semen analysis Testing
Motility, or the number of sperm that are actually swimming, is important for success with natural intercourse and intrauterine insemination, because the sperm need to be able to swim up the fallopian tubes, where fertilization takes place. For IVF, sperm motility is not quite so important since sperm can be injected directly into an egg in the lab with the use of intracytoplasmic sperm injection, or ICSI. However, the sperm that are injected need to be alive, and motility is an accurate marker of this (i.e. if sperm are moving then they are live, although sperm that are not swimming may be alive as well). Decreased sperm motility is sometimes called “asthenospermia”.
IMPORTANT ASPECTS OF SPERM MOTILITY
TOTAL MOTILITY: the total percentage of sperm that are actively swimming (the latest WHO criteria define normal total motility as 40 percent or more)
MOTILITY GRADE: how well (on average) the sperm are swimming. There is significant variability in the reporting of motility grades between labs. The primary distinction to always keep in mind when evaluating motility grades is how many sperm have what is called “progressive” motility, meaning that they have good forward movement. Sperm that are moving but just twitching in place (not moving forward) are not going to be able to swim up the fallopian tubes to where egg fertilization takes place. The 5th edition WHO defines normal progressive motility as 32% or above. We will now describe some of the more common ways that labs denote progressive motility.
Method #1: Average grade of motility
Many labs describe the average grade of how all of the sperm are swimming. This does not really fit in with the WHO classification of percentage of sperm with progressive motility but provides the overall average of sperm. Although there is some variability between labs, degrees of motility are generally defined as:
1) No motility- this is usually defined as either Grade 0 (or Grade D)
2) Sluggish movement with absent or minimal forward progression- this is usually considered to be grade 1 or Grade C
3) Decent sperm activity with forward progression- this is called Grade 2 or Grade B
4) Very strong activity with forward progression- this is generally defined as Grade 3 or Grade A
The last 2 categories (grade 3/grade A and grade 4/grade B) are considered to be sperm with progressive motility. Sometimes grades are given as a range, for example: “Grade 1-2”. For these, essentially the grade would be considered 1.5. Normal average grade of motility is considered to be grade 2 or higher.
Of note, if a man has a motility grade of 1, this does not mean that all of his sperm have non-forward progression. There are still most likely a certain percentage of sperm present with better forward progression and which are capable of traveling up the fallopian tubes (unlike the majority of their fellow sperm in that particular sample).
Method #2- Describing the actual percentage of sperm with progressive motility.
As mentioned previously, the latest WHO criteria define normal progressive motility as 32% or higher. Many labs just provide this information on their reports. Other labs will list each of the 4 categories above (grade 0-3) and list the percentage of sperm that fall into each category (and you then need to do the math to figure out of if the ones with progressive motility add up to 32% or higher). For example:
25% Progressive motility (Grade 3)
15% Slowly progressive (Grade 2)
10% Twitching (Grade 1)
50% Non-motile (Grade 0)
So for this patient, the total motility would be 50 percent (those in grades 1, 2, or 3), while the progressive motility (grade 2 and 3 ) would be 40%.
SPERM AGGLUTINATION
One issue to consider regarding motility is the presence of anti-sperm antibodies (ASAs), which can cause sperm to stick together, thereby decreasing their motility. Most good fertility-specific labs will note on the semen analysis report if significant clumping (also called agglutination) of sperm is seen. Sperm clumping combined with poor motility is suggestive of the presence of ASAs; their presence can be confirmed on repeat semen analysis with direct (immunobead) ASA testing.
More detailed information on anti-sperm antibodies can be found in the above separate section on this website.
The Problem with Using the Standard Semen Parameters
Most physicians rely on sperm density and motility to determine the fertility potential of men. The problem with this approach is that when viewed in isolation, these parameters can provide an inaccurate picture of the actual number of swimming sperm. The reason is that the testicles provide only about 5 percent of the volume of ejaculate that is released during ejaculation. The other 95 percent of the fluid comes from the seminal vesicles and prostate gland, and their fluid production can vary from day to day and has nothing to do with testicular sperm production. In essence, the prostate and seminal vesicles provide the fluid “vehicle” for delivery of the sperm, while the testicles provide the sperm “content.” What determines the actual fertility potential of the semen is the total number of swimming sperm that are present in the entire ejaculate, not the amount of sperm present per unit of semen.
An analogy would be to try to judge how much blue dye is in two different amounts of water. Let’s say you added one cup of concentrated blue dye (representing the sperm) into a gallon of water (lower ejaculate volume) and another one cup into a whole bathtub of water (high ejaculate volume). The amount of dye present is the same in both amounts of water, but the density of dye in the bathtub is going to be significantly lower than in the gallon of water.
The same concept applies to semen. The testicles are adding a certain amount of sperm into the ejaculate, and this amount of sperm is what you are trying to measure. Meanwhile, the ejaculate volume is going to vary depending on the production of fluid by the seminal vesicles and prostate. We want to know the total number of swimming sperm present in the entire ejaculate and not just how concentrated or diluted the sperm is within the ejaculate fluid. The way to determine this total number of swimming sperm is to calculate the total motile count (TMC).
Total Motile Count (TMC) on semen analysis testing
The total motile count (TMC) gets around the problem of fluctuating ejaculate volumes by combining the sperm density, motility, and ejaculate volumes together to provide the total number of swimming sperm that are present in the entire ejaculate.
Some labs report this number under the label “TMC,” while others call it something slightly different, such as “total motile sperm,” “motile sperm count,” or some other similar variation. The key to identifying what the lab is talking about is the unit of measure, which should say “__ million” (or “__ × 106”) for TMC, as opposed to “__ million per cc” (or “__ × 106/cc”) for sperm density. The other differentiation that you need to keep in mind is the “total count,” which is the total number of both swimming and non-motile sperm (and is not as useful a number).
If the TMC is not reported, it can be easily calculated by using the following equation:
(Sperm Density × Ejaculate Volume × Total Motility) ÷ 100
For example, an analysis with an ejaculate volume of 2.0 cc with a sperm density of 30 × 106/cc and a total motility of 50 percent would have a TMC of (2.0 × 30 × 50) ÷ 100 = 30 million sperm.
WHAT IS A NORMAL TOTAL MOTILE SPERM COUNT (TMC)?
In terms of successfully establishing a pregnancy with natural intercourse or IUI, higher numbers of total swimming sperm are better. A TMC of 25 million sperm is getting into the territory of good fertility potential. Men with a TMC of 25 million or above were found to have a 6 times higher chance of successfully conceiving a pregnancy naturally when compared to men with a TMC below this level. [Ayala C. J Androl 1996].
There is some evidence that there may be some additional benefit to increasing levels of motile sperm when conceiving naturally up to a level of 75 million sperm (see below). A 2002 study looked at 940 couples who achieved pregnancy naturally and found a correlation between decreasing time to achieve pregnancy and rising sperm counts up to 55 × 106/cc. Above this level of sperm density there was no additional benefit in how quickly a pregnancy was established. [Slama R. HumReprod 2002] In this study, the average semen volume was 3.14 cc and the average motility was 61.4 percent. Plugging these figures into the equation for calculating TMC produces (3.14 × 40 × 61.4) ÷ 100 = 77 × 106 sperm. So there is some evidence that increasing TMC’s up to around the 75 million range may offer additional benefit for couples trying to conceive naturally.
Summary: Although these numbers are controversial and based on some fairly old studies, a TMC of 25 million or above seems to be a good goal for couples trying to conceive naturally, with some evidence of further benefit for TMC’s of up to 75 million sperm.
WHY CALCULATING TMC IS IMPORTANT
Consider a man who has a sperm density of 30 × 106/cc with an ejaculate volume of 1.1 cc and a total motility of 50 percent. His calculated TMC is 16.5 × 106.
Now, let’s take a second man who has a sperm count of only 6.6 × 106/cc and the same motility, 50 percent, but an ejaculate volume of 5.0 cc. His calculated TMC is the same: 16.5 × 106.
Both of these men have the same number of swimming sperm in their ejaculate. But if the standard WHO definitions are used, the first man would be considered well within the normal range (and would probably not get a recommendation to have a male fertility evaluation, even though he should), and the second man would be considered to have significantly abnormal semen parameters.
MOTILITY GRADE AND TMC
One very important consideration to keep in mind when evaluating TMC is the average motility grade. If most of the sperm are only twitching, then their fertility potential is not going to be very good for natural intercourse or IUI. Therefore, a TMC is considered “normal” only if the average motility grade is 2 or higher.
TOTAL PROGRESSIVE MOTILITY COUNT (TPMC)
Ok, so now that we have figured out TMC, the male fertility scientific community throws in another level of complexity. Instead of calculating the TMC and looking at the average grade of motility, some clinicians prefer to just calculate the total number of swimming sperm which are moving forward progressively. This does make sense in some ways, as this is the population of sperm which generally are going to be able to get to an egg and fertilize it. The calculation is just a little different:
(Sperm Density × Ejaculate Volume × Progressive Motility) ÷ 100
Again, the progressive motility is sometimes listed on the semen analysis report, and sometimes need to be calculated by adding the percentage of sperm with Grade 2/Grade B and Grade 3/Grade A motility.
The “normal” range for TPMC in terms of attempting natural conception has been determined to be 20 million sperm or above by some authors. [Hamilton JAM. HumReprod 2015]. However, it must be noted in this same study that men with very low TPMC’s (<1million) were able to establish a pregnancy up to 25% of the time over a 3 year time period, and that the chances of natural pregnancy were significantly higher when the TPMC was above 5 million sperm. The bottom line is that many couples are able to conceive with sperm counts below the normal TPMC range of 20 million, but higher numbers do increase the chances of success.
Which is better: TMC or TPMC?
I do not think that there is a definitive answer to that question. The TPMC is measuring the total number of sperm which are capable of getting to the egg in the female reproductive tract and this is good information. However, if the overall average sperm grade is taken into account when looking at the TMC, this can provide potentially valuable information as well. Generally, most people use whichever calculation they can do based upon the information provided to them on the semen analysis report. If the lab provides only the average grade of motility, then you have to use TMC. However, if a progressive motility is reported, then you are going to need to use TPMC. Both are perfectly valid, but you just need to understand the difference of what numbers you are looking at (TMC vs. TPMC) since normal values cut-offs for TMC are higher. Often, scientific papers on the topic often use the term “total motile count” to denote both TMC and TPMC, so you need to look into the “Material and Methods” section of the paper to see a description of how the numbers were calculated.
Clinical Impact of Using only Sperm Density and/or Motility in Isolation
As mentioned previously, the semen analysis test is best single predictor of male fertility potential that we currently have today. When utilized correctly, this information can provide important prognostic information on a couple’s chances of success, regardless of whether they are trying to conceive naturally, using IUI, or even IVF/ICSI.
However, sperm counts and motility can be used incorrectly. Just looking to see whether a man’s sperm density and motility are both in the “normal” range as defined by the WHO criteria may be a useful screening device by referring providers to see which men should be referred to a male fertility specialist for further evaluation. However, using these criteria by themselves are not very helpful in predicting if a couple is going to be successful conceiving naturally. This lack of correlation between standard WHO semen parameters and clinical outcomes has been a complaint of clinicians for decades. Multiple studies dating back to 1988 have found that relying just on the defined normal values of isolated parameters such as sperm density and motility as defined by the WHO were not very predictive of that couple’s chances of naturally establishing a pregnancy. [Polansky FF. Fert/Steril 1988] [Van der Steeg JW. FertSteril 2011] [Esteves SE. Urology 2012]
As described earlier, the calculation of the total motile sperm count provides a more accurate assessment of a man’s fertility potential by determining the total number of swimming sperm in the ejaculate. We will now look at the impact of TMC and TPMC on predicting the chances of successfully establishing a pregnancy using natural intercourse, IUI, and IVF.
IMPACT OF TOTAL MOTILE SPERM COUNTS (TMC) AND TOTAL PROGRESSIVE MOTILE SPERM COUNT (TPMC) ON FERTILITY OUTCOMES
Natural Intercourse Outcomes Related to Total Numbers of Swimming Sperm
It makes logical sense that if a man has more and better swimming sperm, that his chances of conceiving a pregnancy naturally would be higher. Therefore, it has always been very frustrating for clinicians that the standard values for sperm density and motility were not particularly useful in counseling couples on their chances of establishing a pregnancy naturally. A 2015 study of 2,476 infertile couples fortunately found that calculating a man’s total progressive motility count (TPMC) seemed to solve these problems and much more accurately predict the chances of a spontaneous ongoing pregnancy. It found that men with a TPMC of <5 million sperm had a significantly lower chance of establishing a pregnancy naturally than a TPMC of 5 million or above. (Hamilton JAM. HR 2015]. Interestingly, even men with extremely low counts (TPMC <1 million) were able to establish a pregnancy naturally about 25% of the time within the 3-year study period. The ability of total motile counts to predict natural pregnancy was also shown by a study of 1,055 couples which again showed sperm density and motility by themselves did not correlate well with outcomes. However, if the sperm TMC was 25 million sperm or above, the chances of natural conception were 6.1 times higher than if the TMC was less than 25 million. [Ayala C. JAndrol 1996]. This cut-off closely follows the generally accepted definition of a normal TMC which is 20 million sperm or above.
Conclusion: Natural conception is possible at even very low sperm counts (TPMC <1million) and sperm count and motility by themselves are not good predictors of success. Increased TMCs are correlated with increased chances of success with natural intercourse. If a couple has been trying for at least a year and they have a TPMC/TMC of <20-25 million sperm, they may want to consider moving on to other treatment options such as IUI or IVF.
Intrauterine Uterine Insemination (IUI) Outcomes Related to Total Numbers of Swimming Sperm
Like for natural intercourse, it intuitively makes sense that IUI success rates would be higher when more motile sperm are present in the ejaculate. Numerous studies have been reported over the years looking at IUI outcomes and how they correlated with a man’s TMC and TPMC. What each study has tried to evaluate is a cut-off point below which IUI is not worthwhile to try and the couple should just consider moving on to IVF.
Pre-Wash vs. Post-Wash: When an IUI is performed, the semen specimen needs to be “washed” in the lab in order to remove potentially detrimental substances (such as bacteria and white blood cells) that are normally screened out by the cervix during natural intercourse. The semen is also concentrated into a smaller volume and the lower quality sperm are selected out. During an IUI, the counts and motility are usually again evaluated after the wash. In general, sperm counts go down and percent motility goes up after a wash, since the sperm of lower quality (and lower motility) are removed. Studies have looked at whether post-wash vs. post wash motile counts are more accurate at predicting IUI outcomes.
Pre-Wash Total Motile Count (TMC) and IUI
Some studies have found that a cut-off for TMC of 5 million sperm or above is recommended for couples wanting to do IUI. [Dickey RP. FertSteril 1999] For example, a 2010 study of 353 couples found an overall clinical pregnancy rate per couple of 28.5% for IUI when the TMC was less than 5 million sperm, as opposed to 44.3% when the TMC was 5 million or above. [Merviel P. FertSteril 2010] However, other studies point to a higher recommended TMC cut-off of 10 million sperm. A 2001 study of 3,479 IUI cycles in 1,039 couples showed a pregnancy rate per cycle of only 2.5% in men with a TMC of less than 5 million, as opposed to 7.1% when the TMC was 5 million or greater. [Van Voorhis BJ. FertSteril 2001] Another study of 308 IUI cycles split couples into 3 groups based upon their TMC. For men with a TMC of less than 5 million, no successful pregnancies were reported with IUI. For men who had a TMC of 5 to 10 million, the pregnancy rate per cycle was 9.5%, and this rose to 17.4% when the TMC was above 10 million. [Cohlen BJ. HumReprod 1998]
Pre-Wash Total Progressive Count (TPMC) and IUI
Multiple studies have looked at TPMC counts pre-wash and their correlation with IUI outcomes. Some early studies recommended a pre-wash TPMCs of 1 million or above to increase the chances of a successful IUI. [Campana A. HumReprod 1996][Huang HY. JAssistReprodGenet 1996]. However, more recent studies suggest that a higher TPMC is associated with increases chances of pregnancy with IUI. A 2011 study looking at 820 IUI cycles in 445 couples found that pregnancy rates/cycle rose from 5.1% in men with a TMC < 5million to 15.1% in men with a TPMC that was between 5 and 10 million sperm. [Nikbakht R. IntJFertSteril 2011]. Similar results were found in a 2014 study of 672 IUI cycles where a pre-wash TPMC of <5 million sperm had a pregnancy rate of 0% as opposed to 12.73% in men with TPMC of between 5 to 10 million sperm. [Zhang E. MaterSociomed 2014].
Post-Wash Total Motile Counts (PWTMC) and IUI
The main problem with post-wash calculations is that these numbers are generally not available for new couples to counsel them on whether they would be good candidates for IUI. Also, there is evidence from studies showing that post-wash TMCs are no better at predicting IUI outcomes than pre-wash numbers. [Luco SM. EurJObstetGynReprodBiol 2014]. However, once a couple has done at least one IUI cycle, post-wash TMC numbers should be available for review and potentially provide additional information which can help guide couples in deciding on whether to try more cycles of IUI. A 2014 meta-analysis of 55 studies found that a PWTMC of 1 million sperm can be used as a cut-off for increased chance of success with IUI. {Ombulet W. ReprodBiomedOnline 2014]. However, a 2009 study of 393 couples found that if the PWTMC was < 5million sperm, then the pregnancy rate per cycle was 5.55%, as opposed to 24.28% if the PWTMC was 5 million or above. [Badawy A. FertSteril 2009].
Conclusion: It appears from the data that the total number of swimming sperm in the ejaculate do correlate with IUI outcomes. Both pre-wash and post-wash numbers can be used to predict the chances of success with IUI. Pre-wash numbers are more clinically useful for couples who have not yet tried IUI, but post-wash evaluations of semen parameters can help to predict the success of future cycles. There is significant controversy as to cut-off ranges for total numbers of swimming sperm below which a couple will be at a significantly increased risk of IUI failure and should just consider going straight to IVF. For pre-wash TMC, the general range is 5-10 million sperm and for pre-wash TPMC, this range falls between 1-5 million sperm. In terms of post-wash TMC, the range below which to consider moving on to IVF falls between 1-5 million sperm. One potential way to look at these numbers is that IUI is possible for couples that fall within these ranges, but to be a good “solid” candidate for IUI from the male side, you would ideally have a pre-wash TMC of 10 million or above, pre-wash TPMC of 5 million or above, or a post-wash TMC of 5 million or above.
IVF/ICSI Outcomes Related to Total Numbers of Swimming Sperm
Not many sperm are needed for IVF/ICSI. The average women undergoing IVF typically has between 5-15 eggs retrieved, so theoretically only this number of viable sperm are needed to inject them. In the early days of ICSI, many clinicians felt that sperm quality no longer mattered because the ICSI process compensated for any defect in sperm quality (so long as enough viable sperm were present to inject each egg). However, many studies have now documented that lower sperm quality can have a negative impact on IVF/ICSI outcomes. Elevated levels of sperm DFI has been shown in a 2017 meta-analysis to negatively impact the outcomes of IVF/ICSI cycles. [Simon L. AsianJAndrol 2017]
Interestingly, a man’s total motile sperm count has also been shown to correlate with a couple’s chances of IVF/ICSI success as well. A 2016 study looking at 518 couples undergoing ICSI looked at the results of men who had a TPMC of < 1 million sperm vs. those with a TPMC of >20 million sperm. The group with a TPMC of >20 million sperm had a higher fertilization rate (84.9% vs. 72.5%) and lower miscarriage rate (17.9% vs. 33.3%) as opposed to men with a TPMC of <1million sperm. [Borges E. Andrology 2016]. Better TPMC’s also predicted the chances of having high quality embryos as well as the number of embryos that developed to the blastocyst stage. This provides further evidence that sperm quality should be optimized prior to a couple proceeding with treatments from the female side, including both IUI and IVF/ICSI.
Sperm Morphology on Semen Analysis Testing
Morphology refers to the number of sperm that have perfectly normal shapes. In my experience, the morphology reading is the one semen analysis parameter that generates the most confusion among clinicians and anxiety among couples. Most of the time this concern is not warranted, as among the three most significant parameters (density, motility, and morphology), morphology is arguably the least important in terms of determining a man’s overall fertility potential. Higher levels of abnormal sperm shapes have been noted in men with infertility [Guzick DS. NEJM 2001] but the prognostic value of morphology reading in clinical practice have been questioned by recent research. However, sperm morphology should not be completely ignored, and efforts to improve the percent of normal shapes as much as possible is recommended.
HOW IS THE Sperm MORPHOLOGY READING DETERMINED on Semen Analysis Testing?
Morphology evaluations take into consideration multiple aspects of a sperm’s shape, including its head, midpiece, tail, and the presence of cytoplasmic droplets. Abnormal sperm morphology is called teratospermia. Using modern guidelines, a lab will evaluate 200 sperm in a specimen and determine what percentage of these sperm have perfectly normal shapes. Some labs just report the percentage of sperm with normal shapes, while others will then break down the reasons why the other sperm were abnormal. An example could look like this:
Normal sperm 5%
Head defects 90%
Midpiece defects 2%
Tail defects 2%
Cytoplasmic droplets 1%
Morphology readings are highly subjective and rely upon the training and experience of the person performing the evaluation. I therefore typically only take into consideration the morphology findings from fertility-specific laboratories (not hospital-based labs).
Sperm MORPHOLOGY CRITERIA
There are several types of guidelines that you may see used for sperm morphology on a semen analysis report, and the report will generally state which of these criteria they are using. The two most commonly used are the World Health Organization guidelines (third edition) and the Kruger strict criteria. You may also see a reference to the fifth edition of the WHO guidelines, which are essentially the same as the Kruger strict criteria.
Kruger Strict Criteria
The most clinically useful morphology criteria is the Kruger “Strict” morphology (which as mentioned above, is the same as the WHO 5th edition criteria). The Kruger criteria were developed in 1986 by a physician named Thinus Kruger and were based upon the evaluation of sperm that had successfully migrated to the cervix following natural intercourse. The original 1986 guidelines used a figure of 14 percent or greater normal forms as the cutoff for “normal” morphology. However, the strictness of the guidelines has changed over the years. [Kruger TF. FertSteril 1986]. The criteria were based on the appearance of sperm which had successfully migrated up to the upper endocervical canal after natural intercourse with the assumption that these sperm had superior functional capacity.
Most fertility-specific labs now use 4 percent or more as their definition of “normal” (as do the latest WHO guidelines), but you will still find some labs that report the old Strict criteria cut-off (14%) or some other normal level (e.g. 8%). I personally use the 4 percent figure as the lower limit of “normal,” although some of the labs in my area still print the higher 14 percent guideline on their official semen analysis reports (a source of endless confusion and anxiety for my patients). I also rarely see Strict morphology readings higher than 10%, so as long as I can get a man’s Strict morphology to 4% or above then I am satisfied with this.
Note: If the Strict morphology is "0%", this does not usually mean that there are absolutely no sperm with a normal morphology. Rather this means that less than 1% of the sperm have completely normal shapes.
World Health Organization Guidelines on Sperm Morphology
Since 1980, the World Health Organization (WHO) has issued guidelines for what it considered “normal” semen morphologies. Over the decades, the criteria for what the WHO considers normal has gotten progressively more rigorous.
WHO Edition Normal Morphology Range
1st Edition (1980) 80.5%
2nd Edition (1987) 50%
3rd Edition (1992) 30%
4th Edition (1999) 14%
5th Edition (2010) 4%
Starting with the 4th edition, the WHO criteria mimicked the Kruger Strict morphology definition of normal being ≤ 14%. In 2010, the 5th edition again changed in step with the Kruger criteria by defining normal as ≤ 4%. In terms of clinical utility, I feel that the WHO 5th edition has the most relevance to fertility outcomes. Many labs still use the WHO 3rd edition (normal ≤ 30%) but I do not find their use particularly meaningful in helping determine a man’s fertility potential.
Other Sperm Morphology Criteria
There are some other morphology criteria that you may occasionally see used by hospital labs, such as the ASCP (American Society of Clinical Pathology) guidelines. I have not found these to be useful and they have almost no data to back-up any clinical relevance to their use.
CONSIDERATIONS TO KEEP IN MIND WHEN LOOKING AT SPERM MORPHOLOGY
I recommend checking sperm morphology during semen analysis testing in couples having fertility problems, but I always like to keep in mind that there are some inherent problems with the testing and interpretation of sperm morphology results.
1) Differences in Preparation and Criteria of Evaluation
For many years, wide variations existed in how slides were prepared and stained in the lab for evaluating sperm morphology. As described above, the criteria for what is considered “normal” have also changed considerably over the years. Most fertility-specific labs have recently tried to take a more uniform and standardized approach to sperm morphology evaluations, but significant variations still exist. I always like to keep this in mind as I see morphology results from different labs, as well as when I read scientific research articles from around the world which try to evaluate the clinical implications of sperm morphology abnormalities. [Gatimel N. Andrology 2017]
2) Subjective Nature of the Evaluation
Counting sperm numbers and calculating what percentage of them are swimming is a fairly straightforward endeavor for trained lab personnel. However, accurately evaluating sperm shapes on a wide range of very stringent morphological criteria is a much more difficult task. Different lab technicians often read the same morphology slides very differently. Wide ranges of inter-lab and intra-lab variations have consistently been found by studies looking at the ability of lab technicians to accurately and consistently evaluate sperm morphology slides. A 2017 review article found that when lab technicians were shown standardized slides for reading, the degree of variation of their findings (called CV, or coefficient of variation) was quite modest for sperm density (19.2%) and sperm motility (15.1%). [Gatimel N. Andrology 2017] However, for sperm morphology, the CV was considerably higher at 80%. Extra intensive training in reading slides was found to be able to bring this variation down, but not below 50%. In addition, the effects of this training appeared to dissipate over time. In one study, if regular follow-up training was not consistently maintained, most lab technicians showed declines in competency after only 6 to 9 months, and only 26% were able to maintain their competency after 40 months.
3) Basic Statistics
There are legitimate questions regarding whether the sample size evaluated for morphology represents a statistically meaningful evaluation of the entire specimen. [Kohn P. EurUrolFoc 2018] Keep in mind that a semen specimen can have more than 100 million sperm in the sample, but typically only 200 of these sperm are evaluated in order to calculate a Kruger strict Morphology. From a basic statistics standpoint, if only 0.0002 percent of the sperm are being evaluated, it’s hard for me to see a significant clinical difference between a sample that shows 5 percent normal forms and one that shows 3 percent normal forms when samples sizes are so small.
CLINICAL IMPACT OF Sperm MORPHOLOGY
The role of sperm morphology and its impact on male fertility fall into 2 primary areas of concern:
#1) The impact on the ability to conceive and maintain a healthy pregnancy
#2) The concern regarding an increased risk of genetic abnormalities or health problems for subsequent offspring
[Note: The recommendations made in this section (and whole website) represent the best overall conclusions taking from evaluating the scientific literature as a whole. Studies can be found on most any topic in male infertility that contradict the conclusions/recommendations of this website. This is especially true regarding controversial topics such as sperm morphology, due to the wide range of different criteria that have been used over the years, as well as the large inter-lab variability in the consistency of the reading of slides. The following recommendations are made on the best available data derived from meta-analyses of these studies as a whole.]
The impact of abnormal sperm morphology on GENETIC AND/OR HEALTH RISKS IN FUTURE CHILDREN
Many couples look at the results of their semen analysis test and see “98% head defects” and panic. If the sperm DNA is stored in the sperm head, and the vast majority of these sperm heads are abnormal, does using these sperm have a higher chance of passing on potential genetic problems to future children? Fortunately, the answer to this question appears to be “No”.
SPERM ANEUPLOIDY
Sperm aneuploidy is a term which describes abnormal levels of chromosomal abnormalities in sperm, and can be evaluated with specialized testing, such as FISH (fluorescent in situ hybridization). The concern is that if genetically abnormal sperm fertilize an egg, then the resulting embryo (and subsequent child) can have genetic abnormalities as well. A 2017 review article on morphology in the journal Andrology [N. Gatimel. Andrology 2017] found that most studies showed a slightly elevated rate of sperm aneuploidy in men with teratospermia, but this rate was not significantly different than men with only low sperm counts and motility. In other words, poor semen parameters of any kind were associated with mildly increased rates of sperm aneuploidy, but that abnormal morphology did not represent a higher risk factor than other parameters. Also, these elevated rates of aneuploidy were only seen in ejaculated sperm but did not appear to persist after egg fertilization. It is known that the unfertilized egg has very efficient screening mechanisms for weeding out defective sperm and preferentially letting normal sperm through its outer layers to start the fertilization process. Another meta-analysis of studies found no association between sperm morphology and the rates of sperm chromosomal abnormalities. [Sun F. ReprodBiolEndocrinol 2006] In addition to these findings, nature seems to be very efficient at identifying embryos that do have genetic abnormalities and not allowing them to progress (i.e. resulting in spontaneous early miscarriage). Of course, these mechanisms are not 100 percent efficient, but rates of health problems and birth defects do not seem to be higher in the children of men with lower morphology readings, whether the pregnancy was established by natural intercourse, inseminations, or standard IVF. There is some concern about whether there is an increased risk of birth defects when using ICSI, since this technique bypasses some of the egg’s natural screening mechanisms. However, nature’s efficiency at removing genetically defective embryos again helps to ensure that the vast majority of ICSI babies are born completely healthy and normal. Also, when ICSI is used, the lab is usually able to carefully select individual sperm for use that have completely normal shapes. Therefore, it is generally considered that elevated levels of sperm morphology defects are not considered a significant risk factor for having subsequent children with genetic abnormalities, birth defects, or other health problems. Only a few rare exceptions exist to this general rule, such as in men with globozoospermia (see below), double heads, and multiple tails- some of these will be reviewed in more detail below. [Sun F. ReprodBiolEndocrinol 2006]. Higher rates of sperm aneuploidy have been found in these relatively rare sperm with highly abnormal shapes and these should not be used with IVF/ICSI.
RARE SPERM MORPHOLOGIC ABNORMALITIES
Globozoospermia
There is a rare type of teratospermia called globozoospermia, in which sperm have small round heads that lack an acrosome cap (which is needed for the sperm to penetrate an egg). A good fertility-specific lab will usually indicate on the semen analysis report if all of the sperm are lacking an acrosome cap. If all of the sperm are affected, then this is called Total Globozoospermia Syndrome. The cause of this syndrome is not known but there are felt to be genetic factors involved in some men. Pregnancy rates in patients with globozoospermia are usually quite low, even with the use of IVF/ICSI. Specialized IVF laboratory techniques, such as assisted oocyte activation (AOA), have been found to potentially improve fertilization rates in some of these cases.
Microcephalic Sperm
Microcephalic sperm have head sizes that are <2.5 micrograms in width and <3.5 micrograms in length. Acrosome abnormalities are common in these sperm. Microcephalic sperm are associated with elevated levels of sperm DNA fragmentation, and tend to have low fertilization and pregnancy rates, even with the use of ICSI.
Pin Head Sperm
Pin head sperm have tails, but no head. The cause of these are not known. If used with ICSI, fertilization of the egg can occur, but not embryo progression (i.e. a viable pregnancy cannot be established with the use of these sperm).
Macrocephalic Sperm
Macrocephalic sperm have large irregular heads, and often multiple tails (average 3.6 tails per head). If all of the sperm are affected, this is called Macrocephalic Sperm Syndrome. Macrocephalic sperm generally have high rates of sperm aneuploidy. Genetic causes have been identified in some men, as has the use of sulfasalazine for inflammatory bowel disease. Use of these sperm for ICSI is contraindicated due to the concern of passing on genetic abnormalities to the resulting offspring.
IMPACT OF abnormal sperm MORPHOLOGY On FERTILITY AND FERTILITY TREATMENT OUTCOMES
Concerns have been raised as to the extent to which elevated levels of sperm morphologic abnormalities can decrease a couple’s chances of conception. Various hypotheses have been proposed as to how sperm morphology could potentially impact fertility, including impaired sperm passage through the cervical mucus as well as decreased binding to the egg zona pellucida during egg fertilization. However, these findings are controversial, and the exact mechanism of morphologic defects leading to reduced fertility is not known. More concrete (though still controversial) data is available on the degree to which morphology plays a role in fertility outcomes based on different fertility treatment options utilized by couples.
Natural Intercourse Outcomes and Sperm Morphology
The use of strict criteria was originally evaluated in its relationship with IVF outcomes. [Kruger TF. Urology 1987]. Most studies on strict morphology outcomes relate to its use with predicting IUI and IVF success rates. However, a 2015 study of 1,177 couples trying to conceive naturally showed no relationship between sperm morphology and the chances of establishing a pregnancy. [Hamilton JAM. HumReprod 2015]. A review of the literature in 2016 “suggests that patients with abnormal sperm morphology alone should not be precluded from attempting natural conception before undergoing assisted reproduction. [Shabtaie SA. CurrUrolRep 2016] Sperm counts and motility are generally considered to have a much larger impact on natural intercourse success rates, and the establishing of healthy pregnancies are not necessarily negatively impacted even with very low (<1% normal) strict morphologies.
Intrauterine Insemination (IUI) Outcomes and Sperm Morphology
Similar to natural intercourse, sperm counts and motility are generally considered much more important parameters for success than sperm morphology. In the past, many fertility specialists felt that if sperm morphologies were decreased, then IUI success rates were low and couples should consider just move on to IVF. This viewpoint seemed to be supported by early research on the topic such as the 2001 meta-analysis looking at 9 studies on the topic. [Van Waart J. HumReprodUpdate 2001]. In this review, 6 of the 9 studies showed a negative impact of low sperm morphology on IUI outcomes. However, most of these studies did not look at the morphology as a factor by itself, but rather found that it was correlated only when other factors were present, such as a low total motile sperm count as well. [Lockwood GM. Andrology 2015]. More recent studies on the topic, however, have shown that morphology does not appear to impact IUI outcomes as long as sperm counts and motility are good. An example is a 2015 study looking at 856 IUI cycles in 408 couples, where pregnancy rates per cycle were 15.7% for men with a strict morphology of 4% or less as opposed to 13.9% when the morphology was 5% or higher. [Devereau NE. FertSteril 2014]. Even for patients with a very low morphology of 0-1%, pregnancy rates per cycle were 21.4%. Similar findings were seen in a 2019 study of 984 IUI cycles in 501 couples, which found a clinical pregnancy rate/cycle of 12.3% in men with a morphology of less than 4% in comparison to 13.0% for men with morphology of 4% or above. [Patel P. JUrol 2019]. In this study, there was also noted to be no change in live birth and miscarriage rates in men with lower morphology. A meta-analysis of 20 studies on the topic in 2018 evaluated 41,108 IUI cycles and noted no significant difference in pregnancy rates when the strict morphology was <4% (12.1%) vs. 4% or above (14.2%). [Kohn TP. JUrol 2018]. This same review also noted no difference in IUI pregnancy rates with very low strict morphologys of 0% (13.9%) when compared to men with a strict morphology of 1% or higher (14.3%).
It is becoming increasing apparent that sperm morphology does not seem to correlate with IUI outcomes, and the data suggests that low morphology should not significantly decrease the chances of success in couples with otherwise good total motile sperm counts.
Standard IVF vs. IVF/ICSI Outcomes and Sperm Morphology
The original studies on the Kruger strict morphology were performed looking at the impact on outcomes with standard IVF. At that time, a normal strict morphology was defined as ≥ 14%. Couples undergoing standard IVF showed higher fertilization rates with a morphology of 14% or above (88.3%) vs. men with a morphology of less than 14% (49.4%). [Kruger TF. Urology 1987]. Pregnancy rates/embryo were also higher in men with a morphology of 14% or higher (51.5%) as opposed to a morphology less than 14% (18.5%)
Since that time, however, conflicting data has emerged on the impact of strict morphology outcomes on the outcomes of standard IVF. A review of 10 studies in 1998 found that pregnancy rates/cycle were higher in men with a strict morphology of >4% (26.0%) as opposed to <4% (15.2%) with standard IVF. [Coetzee K. HumReprodUpdate 1998]. Several other studies soon after confirmed these findings that decreased strict morphology seemed to have a negative impact on standard IVF outcomes. [Marnet B. IntJAndrol 2000][Gunalp S. HumReprod 2001][Menkveld R. HumReprod 2001]. However, a meta-analysis in 2011 looked at 4 studies that they felt fit the adequate criteria for evaluation, with findings suggesting that standard IVF outcomes were not impacted by low sperm morphologies. [Hotaling JM. FertSteril 2011]
In terms of IVF/ICSI, the data seems appears fairly clear that sperm morphology does not negatively impact outcomes. A study of 3,676 IVF/ICSI cycles in 2015 found that success rates were not decreased when sperm morphology rates were low. [van den Hoven L. Andrology 2015]. The 2011 meta-analysis study mentioned in the last paragraph also found that IVF/ICSI success rates were unchanged by sperm morphology. [Hotaling JM. FertSteril 2011]
Conclusion: A review of available literature in 2017 concluded that sperm morphology does not appear to impact the outcomes of IVF/ICSI. [Gatimel N. Andrology 2017]. In regard to standard IVF, this paper found that the majority of the published studies support the findings that decreased strict morphology may have a negative impact on outcomes. Based on these findings it can be recommended that at this time, men with an abnormal strict morphology (<4%) may benefit from the use of ICSI during an IVF cycle.
SUMMARY OF SPERM MORPHOLOGY
The clinical relevance of the strict morphology on non-IVF treatments seems to continue to diminish as further research on the topic accumulates. A low strict morphology (<4%) can still serve as a screening tool for deciding which men may benefit from an evaluation by a male fertility specialist, and efforts should be made to try and improve the morphology as much as possible by optimizing the environment for sperm production. However, in terms of natural intercourse and “low-tech” treatments from the female side (such as IUI), the predictive value of sperm morphology appears to be minimal, and the current data seems to indicate fairly clearly that a low morphology does not mean that a couple would necessarily benefit by moving directly on to IVF.
Ejaculate Volume on Semen Analysis Testing
The role of the semen is to efficiently transport sperm to the cervix while protecting them from the harsh, acidic environment of the vagina. A standard semen analysis almost always includes an assessment of ejaculate volume, color, and time for liquefaction. Some labs will also routinely check pH and sometimes semen fructose. These semen parameters will make more sense if you know the relative contributions of the testicles, seminal vesicles, and prostate to the ejaculate fluid.
1) Testicles: 0.1 cc. The testicles supply the sperm and a small amount of fluid (about 5–10 percent of the total ejaculate volume). Because this is such a small percentage of total volume, men who have had a vasectomy, in which the flow of fluid from the testicles is blocked, do not notice any difference in ejaculate volume.
2) Seminal vesicles: 1.5 cc, alkaline pH. The seminal vesicles supply the bulk of the fluid volume (about 65–70 percent). Seminal vesicle fluid causes the ejaculate to coagulate, and also provides fructose as an energy source for the sperm. Since the majority of fluid comes from the seminal vesicles, the overall pH of a normal ejaculate is alkaline.
3) Prostate: 0.5 cc, acidic pH. The prostate gland produces around 25–30 percent of seminal fluid. Prostatic fluid provides enzymes that cause the coagulated semen to liquefy, thereby releasing the sperm into the cervical mucus. Typically, the ejaculate liquefies completely within twenty to thirty minutes when evaluated in the lab.
4) Bulbourethral glands (Cowper’s glands): The bulbourethral glands are pea-sized glands, located at the base of the penis, that open into the urethra. With arousal, these glands produce a small amount of a clear, salty, viscous secretion called pre-ejaculate. The pre-ejaculate serves to lubricate the urethra and neutralize any acidic urine that may remain within the urethra from the last urination. Although the bulbourethral glands do not typically serve as a reservoir for sperm, their fluid can pick up sperm dwelling within the urethra from the last ejaculation and carry them out.
So, to summarize the relative contributions of these structures to the volume of semen that comes out at the time of ejaculation:
1) Testicles: 5 percent of total ejaculate volume
2) Seminal vesicles: 65–70 percent of total ejaculate volume
3) Prostate: 25–30 percent of total ejaculate volume
NORMAL EJACULATE VOLUME on semen analysis testing
The fifth edition of the WHO criteria list a normal ejaculate volume as 1.5 cc or more.
However, I personally use 1.0 cc as the cutoff for what I consider normal, since in my clinical experience I have found extremely low rates of ejaculatory problems (such as retrograde ejaculation) in men with ejaculate volumes between 1.1 cc and 1.5 cc.
I do not really worry about higher than normal ejaculate volumes, unless there is a concern about urine leaking out with ejaculation. This is extremely rare and would likely only occur in men with significant neurologic issues. Presumably the semen pH would be lower (see below) since urine is acidic.
Low ejaculate volumes can cause problems with fertility- for more detailed information on this, please see "Reversible Semen Analysis Factors" in the Sperm Boot Camp section of this website.
ABNORMAL EJACULATE VOLUME on Semen Analysis Testing
Most labs report a normal ejaculate volume as above 1.0 cc to 2.0 cc, and the 2010 WHO guidelines use 1.5 cc as the lower limit of normal. I personally use the 1.5 cc cutoff level for normal, but am usually not too worried with an occasional borderline low volume (1.1-1.4cc) volume, especially if the abstinence time is low (2 days or less). I frequently see borderline low ejaculate volumes on a man’s first semen analysis, and I feel that this is often due to the man feeling nervous and inhibited with the novel experience of providing a specimen sample in a cup. For the subsequent analysis I have them make sure to aim for 3-4 days of abstinence time and come in well hydrated (since hydration status can potentially play a role as well). On their second analysis they know what to expect and are usually a little more comfortable with the situation, and the ejaculate volume is usually in the normal range (≥ 1.5cc). If it is persistently low, however, then further evaluation would be warranted (see below).
Labs often report an upper limit on normal volume (for example, 6.0 cc), but I am not usually concerned about ejaculate volumes higher than this. If I see an ejaculate volume that is very high (for example, 20 cc) in someone with a neurologic disorder or someone with prior urethral surgery, then I get suspicious that the ejaculate may contain some urine that is leaking out with the ejaculate (in which case the pH should be acidic, reflecting that acidity of the urine). This is a rare finding, though, and high ejaculate volumes are typically fine, just representing a more robust production of fluid volume by the prostate and seminal vesicles.
A more worrisome finding is to have a low ejaculate volume. The most common reason for a low ejaculate volume is a failure to collect the entire specimen in the collection cup. Sometimes the lab will report if part of the specimen was lost during collection which is helpful. However, if the ejaculate volume is low and there is no report of missed a specimen collection, I always ask the patient if they collected all of the specimen since the lab does not always report it (and the patient does not always tell the lab).
POSSIBLE CAUSES OF LOW EJACULATE VOLUME
If none of the specimen was missed on collection, then the most common reasons for a low ejaculate volume include:
1) Short abstinence interval. Before providing a specimen for semen analysis, you should abstain from sexual activity of any kind, including masturbation, for two to five days. If you abstain for less than two days, the seminal vesicles may not have time to fully recharge, and this can contribute to a lower than normal ejaculate volume.
2) Low testosterone levels. The functions of the seminal vesicles and prostate gland are influenced by testosterone levels. Low testosterone levels can lead to a decrease in ejaculate volume in some men.
3) Retrograde ejaculation. This is a situation where the bladder neck does not tighten down as it should during ejaculation, and either all or part of the ejaculate flows backward into the bladder. Post-ejaculatory urinalysis testing can be used to evaluated for the presence of retrograde ejaculation. See the “Ejaculatory Dysfunction" section of this website for more details.
4) Ejaculatory dysfunction. This occurs when the muscular vas deferens does not properly transport the sperm from the epididymis to the urethra. A severe form of this, in which no fluid comes into the urethra at all, is called anejaculation. Common reasons for ejaculatory dysfunction include neurologic problems (such as spinal cord injury or diabetes mellitus) and certain medications (such as antidepressants). See “Ejaculatory Dysfunction” section later for more information using the link above.
5) Ejaculatory duct obstruction (EDO). In men with EDO, the fluid from the vas deferens and seminal vesicles is blocked from entering the urethra. This can be either a partial or complete blockage. See “Ejaculatory Dysfunction" section (link above) for further information on EDO.
6) Seminal vasculopathy. The seminal vesicles can become dysfunctional and fail to contract, leading to decreased amounts of seminal vesicle fluid entering into the ejaculate. Neurologic problems, such as diabetes mellitus, are the most common cause of seminal vasculopathy.
7) Dehydration. If a man is very dehydrated at the time of specimen collection, it can potentially play a role in decreased ejaculate volumes, although this has not been studied extensively. For men with a borderline ejaculate volume, I recommend that they come in well hydrated for any follow-up semen analysis testing, drinking enough fluids to keep the urine pale yellow (generally a good sign of adequate hydration).
PYOSPERMIA
ELEVATED WHITE BLOOD CELLS IN THE SEMEN
All men have some white blood cells (WBCs) present within their semen, as they play a role in maintaining a good, healthy environment for sperm. When too many WBCs are seen in the sperm, it is called pyospermia and represents an elevated level of inflammation within the genital ducts which can be damaging to sperm. This inflammation can be due to either an infection (bacterial, viral, fungal) or to a non-infectious cause (for example, nonspecific inflammation of the prostate or epididymis). Only about 20 percent of asymptomatic genital duct inflammation seen in male infertility patients is due to an infection.
You may see WBCs reported on a semen analysis in one of two ways. When they are reported as numbers per cc, I consider “normal” to be 1 million/cc or under. When they are reported as number of WBCs seen under a tabletop microscope, I consider “normal” to be 10–15/hpf or less. However, the laboratory diagnosis of pyospermia can sometimes be difficult, since immature sperm cells look very similar to WBCs in a semen specimen (both of which are referred to as "round cells").
ROUND CELLS VS. WHITE BLOOD CELLS
As mentioned above, WBCs on semen analysis testing look very similar to immature sperm cells, and the two cannot be differentiated with the standard staining techniques that labs generally use to evaluate sperm morphology. The term “round cells” is sometimes used to denote cells that could be either WBCs or immature sperm cells. Elevated numbers of immature sperm cells are more often seen in men with low sperm counts, and less commonly in men with normal sperm production.
Special laboratory techniques are available that can differentiate WBCs from the immature sperm cells, but these techniques are usually only performed by fertility-specific labs. Most hospital labs (and some fertility-specific labs) do not perform this more advanced testing and only report the number of round cells that are present. This presents a potential problem when the number of round cells is elevated, since the clinician does not know if they represent potentially harmful WBCs or relatively harmless immature sperm cells.
If you encounter this type of problem, the best solution is to find another lab in the area that offers the specialized testing to differentiate WBCs from immature sperm cells (examples include peroxidase staining [also called an Endtz test], Wright staining, and immunohistochemical techniques). The second-best option is to treat with antibiotics and anti-inflammatory medications, knowing that there is a two-thirds chance that you are taking these medications for no reason (i.e., the round cells were actually just immature sperm cells). This is a less-good option since antibiotics can cause side effects, increase the risk of diarrheal disease, and select for the growth of antibiotic-resistant bacteria within your body. Semen cultures are rarely helpful, since contamination of specimens by non-harmful bacteria present at the very end of the urethra is extremely common.
FERTILITY IMPACT OF PYOSPERMIA
A normal by-product of the body’s metabolic processes is the creation of reactive oxygen species (ROS), also called free radicals (for more information, see the "Oxidative Stress" section of this website).
Under normal circumstances the body is very well equipped to clean up these ROS before they can cause any damage to cells, tissues, or organs. Small amounts of ROS are produced by the sperm themselves, but white blood cells generate very large amounts of these damaging substances. In pyospermia, with its elevated levels of white blood cells, the increased amounts of ROS can overwhelm the body’s cleanup mechanisms, resulting in oxidative stress. Sperm are especially sensitive to oxidative stress-related damage because of the compact structure of their DNA (genetic material), their natural lack of antioxidants, and their inability to effectively repair damage.
Pyospermia/inflammation has been associated with an increased risk of several male reproductive problems, including:
1) Decreased sperm density, motility, and morphology
2) Elevated levels of sperm DNA fragmentation
3) Decreased pregnancy rates and poor embryo progression with IVF
4) Inflammation-related formation of anti-sperm antibodies
EVALUATION OF PYOSPERMIA
Clinically significant pyospermia is typically considered to be a white blood cell count over 1 million per cc or a WBC count of over 10–15 per high power field (hpf) (different labs use different methods for counting WBCs).
It is important to distinguish between asymptomatic pyospermia and symptomatic pyospermia. In asymptomatic pyospermia, a man’s semen analysis shows elevated WBC, but he has no symptoms.
Men with symptomatic pyospermia have elevated levels of semen WBCs in addition to symptoms of inflammation, which can include:
1) Discomfort in the scrotal area or in the perineum (the region behind the scrotum where the prostate gland sits)
2) Urinary symptoms, such as burning with urination, needing to urinate frequently, discomfort with urination, or cloudy foul-smelling urine
3) Urethral discharge (discolored fluid draining from the end of the penis)
In patients coming in for fertility-related reasons, asymptomatic pyospermia is much more common than symptomatic pyospermia. If there are symptoms associated with the elevated WBC count, then the risk of an active infection is significantly increased, and urine testing is indicated. Possible tests include a urinalysis, a urine culture, and/or screening for sexually transmitted diseases such as chlamydia and gonorrhea (STDs typically can be diagnosed by testing the urine, but sometimes a urethral swab is needed).
Diagnostic codes that can be used for these tests include:
Diagnosis ICD-10 Code
Acute cystitis N30.0
Chronic cystitis N30.2
Prostatitis N41.9
STD screening Z11.3
TREATMENT OF ASYMPTOMATIC PYOSPERMIA
As mentioned above, most men being evaluated for fertility who are found to have elevated levels of WBCs in their semen have asymptomatic pyospermia. In about 80 percent of these cases, the pyospermia is from non-infectious causes, meaning that somewhere in the genital duct system (most commonly the prostate) there is some inflammation not caused by an infection. Asymptomatic prostatitis, or prostate inflammation, is fairly common; usually no cause can be found.
Because most men with pyospermia do not have an infection, some fertility specialists choose not to treat them with antibiotics, and instead rely only on only anti-inflammatory medications to clear the WBCs from the genital tract. I prefer not to miss the 20 percent of men whose pyospermia is the result of an infection, and so I usually treat all cases of pyospermia with a combination of antibiotics and anti-inflammatory medications. It generally takes several weeks for these medications to effectively penetrate and treat the genital duct system.
The regimen I typically use is:
1) Antibiotic (doxycycline 100 mg twice a day) for three weeks. Sometimes a longer course (6 weeks or longer) is needed, or switching to another medication (such as ciprofloxacin 500 mg twice a day).
2) Anti-inflammatory medication while taking the antibiotic; I generally recommend naproxen (Aleve) 220mg, 2 pills by mouth twice a day (with breakfast and dinner).
Naproxen can be bought over the counter inexpensively. There is also a wide array of prescription anti-inflammatory medications that can be taken instead of ibuprofen, such as oxaprozin (Daypro) and celecoxib (Celebrex). These have the advantage of needing to be taken less often than naproxen. However, they are significantly more expensive and are not felt to be more effective than ibuprofen in their anti-inflammatory effect. Medications such as celecoxib are also marketed as having less gastrointestinal side effects, but they have been associated with an increased risk of cardiovascular events with prolonged use. Before taking anti-inflammatories, it is important to check with your doctor if you have a history of stomach ulcers, gastroesophageal reflux disease (GERD), or decreased kidney function or kidney disease. Mild stomach upset can be managed with a trial of over-the-counter Pepcid or Prilosec. However, for persistent or worsening symptoms, you should stop your anti-inflammatory and check with your doctor.
It takes time for inflammation to subside, so I recommend a repeat semen analysis four to six weeks after finishing treatment for pyospermia. I’m looking for a normal WBC level, under 1 million per cc. If WBC levels are decreasing but are still above normal at the time of the retest, then a third semen analysis ten to twelve weeks later may be indicated.
TREATMENT OF SYMPTOMATIC PYOSPERMIA
Treatment of symptomatic pyospermia is dependent on the results of the diagnostic testing that was performed, such as a urinalysis or urine culture. Once the treatment is complete and the symptoms have cleared up, semen analysis is repeated six to eight weeks later. If the symptoms do not clear up, then further evaluation and treatment by a urologist is indicated.
For chlamydia or gonorrhea, the latest guidelines from the Centers for Disease Control recommend a single dose of one of the following:
1) Ceftriaxone 250 mg by injection or
2) Cefixime 400 mg by mouth or
3) Ceftizoxime 500 mg by injection or
4) Cefoxitin 2 g by injection along with probenecid 1 g by mouth or
5) Cefotaxime 500 mg by injection
plus one of the following:
1) Azithromycin 1 g by mouth in a single dose or
2) Doxycycline 100 mg by mouth twice a day for seven days
If a sexually transmitted disease such as chlamydia or gonorrhea is found, the female partner should also get tested by her ob-gyn. The couple should use a condom or practice abstinence until both partners have completed their courses of treatment, to prevent spread of the infection back and forth between them.
If the urine test comes back as positive for a bacterial urinary tract infection (UTI), then the choice of antibiotic should be determined by what antibiotic the culture says the bacteria are sensitive to. Typically a ten-to-fourteen-day course of antibiotics is indicated for men, though a longer course (three to six weeks) may be necessary if the doctor suspects prostatitis as well.
Urinary tract infections are much less common in men than in women. If a man is diagnosed with a symptomatic urinary tract infection, he should see a general urologist for a basic evaluation (including a non-invasive ultrasound test to make sure that he is adequately emptying his bladder).
PERSISTENT PYOSPERMIA
If significant pyospermia persists following adequate treatment, then further testing is recommended to make sure that some hidden source of inflammation is not being missed, including potentially dangerous but rare problems like prostate cancer.
Potential recommended testing includes:
1) Chlamydia and gonorrhea testing by urine test or urethral swab (if not previously done). Diagnosis code (STD testing): Z11.3. These sexually transmitted diseases can be entirely silent and without symptoms in some circumstances.
2) Transrectal ultrasound (TRUS). Diagnosis code (prostatitis): N41.9. This radiologic test provides good visualization of the prostate and seminal vesicles, and can look for abnormalities in the area such as stones or abnormal masses. Not all radiology centers offer TRUS, so you may need to call around and find one that does. Urologists also typically offer TRUS in their office.
3) Prostate-specific antigen (PSA). Diagnosis code (PSA screening): Z12.5. PSA is a screening test for prostate cancer. It is typically used in conjunction with a digital rectal exam. Prostate cancer is rare in younger men, but I have seen it in men as young as thirty. A tumor in the prostate can lead to inflammatory changes and pyospermia, depending on its location within the gland. Further evaluation by a urologist is indicated for abnormalities seen on the PSA test or ultrasound imaging. If the testing all comes back as normal, then I would recommend yearly PSA and digital rectal exam with a primary care physician or general urologist.
Semen Cultures
Some physicians recommend checking a semen culture in cases of persistent pyospermia, looking for aerobic and anaerobic bacteria, as well as for other organisms such as chlamydia, gonorrhea, mycoplasma, and ureaplasma. (Treatment for mycoplasma or ureaplasma is two weeks of doxycycline combined with four weeks of ciprofloxacin.) The problem with semen cultures, however, is that they are notoriously inaccurate due to the high rates of contaminated specimens. Even with thorough washing of the hands and penis with antibacterial soap prior to semen collection, the end of the urethra closest to the penile opening is still colonized by bacteria that can contaminate the specimen (but which generally do not cause fertility problems or pyospermia). In some circumstances, such as persistent pyospermia despite treatment, a semen culture might reveal antibiotic-resistant bacteria that may respond to an alternate antimicrobial regimen. However, the high rates of specimen contamination typically make interpretation of the results difficult. I recommend ordering antimicrobial sensitivity testing in these circumstances, so that any bacteria that are grown are tested for susceptibility to different antibiotics. If multiple organisms grow from the culture, then this usually represents a contaminated specimen. If a single organism grows that is resistant to the antibiotics that were used in the past, this information can help guide further treatment.
Managing Persistent Pyospermia
Persistent pyospermia sometimes cannot be eliminated completely. If the tests described above don’t reveal any worrisome findings, then I put the man on antioxidants for at least three months before ordering a test for oxidative stress (See “Antioxidants and Oxidative Stress” section for more details).
If the oxidative stress test results are normal, then the impact of the pyospermia on fertility should be minimal. However, if the results are abnormal, then sperm washing can be used to decrease the impact of the WBCs. WBCs have an increased impact on sperm quality the longer that they are in contact with the sperm. Therefore, immediate sperm washing after ejaculation (combined with intrauterine insemination) can minimize the time the WBCs are in contact with the sperm, possibly limiting oxidative stress damage. This is especially true if the inflammation is felt to be in the prostate region, where the sperm spend less time, though it may be less effective for epididymal inflammation, since the sperm typically spend seven to fourteen days within the epididymis during the normal maturation process.
SEMEN PH AND SEMEN FRUCTOSE
In normal circumstances, ejaculate is alkaline (with a pH of 7.5 or more), since the alkaline fluid produced by the seminal vesicles is the largest contributor to ejaculate volume. The seminal vesicles also produce fructose. So when the ejaculate has a basic pH and contains fructose, that indicates that fluid from the seminal vesicles is making it into the ejaculate. This is important because the fluid from the seminal vesicles causes sperm to coagulate, keeping them near the cervical opening and protecting them from the harsh vaginal environment.
When the semen is acidic (pH of less than 7.5) and/or there is no fructose in the ejaculate, this is generally indicative of a blockage at the level of the ejaculatory ducts (which can occur is isolation, as well as associated with other genital duct abnormalities such as congenital bilateral absence of the vas deferens). For more information on this, please see "Reversible Semen Analysis Factors" in the Sperm Boot Camp section of this website (although some of the causes of obstruction are not technically "reversible" but can be bypassed in some circumstances).
Acidic Semen pH or Fructose Negative Testing on Semen Analysis
The pH of the ejaculate is normally alkaline, with a pH of 7.5 or higher (the seminal vesicles produce a large volume of alkaline fluid, substantially more than the acidic fluid produced by the prostate). The seminal vesicles also produce fructose. So when the ejaculate has an alkaline pH and contains fructose, then fluid from the seminal vesicles is successfully entering the ejaculate.
The ejaculatory ducts carry the fluid from the vas deferens and seminal vesicles to the urethra, where it is joined by the prostatic fluid. Complete blockage of the ejaculatory ducts blocks all fluids coming from the testicles and seminal vesicles, so that only fluid from the prostate enters into the ejaculate. (See “Ejaculatory Dysfunction” section in this website for more information on ejaculatory duct obstruction.)
Therefore men with complete ejaculatory duct blockage typically have no sperm at all in the ejaculate (azoospermia), low ejaculate volume (less than 1.0 cc), acidic semen pH (under 7.5) and no fructose in the semen.
Other potential causes for abnormalities in semen pH and fructose include:
1) Lab error. Because of this possibility, when the results of the semen analysis don’t match up with what seems to be going on in the man’s body, I recommend repeat testing to rule out a simple error.
2) Incomplete collection. The last part of the semen that is ejaculated contains most of the seminal vesicle fluid. So if the last part of the specimen wasn’t collected in the container, the results could come back as abnormal.
3) Seminal vesicle inflammation. This usually causes elevated levels of semen white blood cells.
Of note, many fertility experts feel that semen fructose testing should never really be needed, since low volume, a complete lack of sperm, and an acidic pH should be enough to clearly indicate the presence of ejaculatory duct blockage or absence.
Semen Color on Semen Analysis Testing
This assessment is highly subjective and rarely clinically useful. One exception is with complete ejaculatory obstruction, where the semen is often entirely clear.
Semen Liquefaction and Semen Viscosity on Semen Analysis Testing
Enzymes in the prostatic fluid cause the coagulated sperm to gradually liquefy, thereby facilitating release of the sperm into the cervical canal. The rate at which the semen liquefies is often measured as viscosity (a normal value is under 2) or liquefaction time (normal times range between 5 and 25 minutes, with an average around 20 minutes; abnormally long liquefaction times are generally defined as more than 30 minutes).
Abnormal semen liquifaction/viscosity is a rare cause of fertility issues. For more detailed information on this topic, please see "Reversible Semen Analysis Factors" in the Sperm Boot Camp section of this website.
ABNORMAL SEMEN LIQUEFACTION AND VISCOSITY
The fluid from the seminal vesicles causes seminal fluid to stick together, or coagulate, in order to hold the sperm near the cervical opening and protect them from the harsh vaginal environment. The enzymes in the prostatic fluid then cause the semen to gradually liquefy, thereby allowing the sperm to enter the cervical canal. The rate at which the semen liquefies is often measured as viscosity (with a normal result being under 2) or liquefaction time (with normal ranging between 5 and 25 minutes).
Sometimes a semen analysis will show a prolonged liquefaction time (more than 30 minutes) or abnormal viscosity (over 2). In most circumstances, a repeat analysis—especially one performed at a fertility-specific lab—will show normal results, so nothing further needs to be done.
However, a persistently abnormal finding could signify one of the following problems:
1) Dehydration. Dehydration can lead to increased semen viscosity. As noted above, I recommend repeat testing with attention to proper hydration beforehand. A good goal is to drink enough to keep the urine pale yellow.
2) Infection or inflammation. In some circumstances, infection or inflammation of the genital duct (especially of the prostate) can increase semen viscosity. High levels of white blood cells (pyospermia) are typically present if there is an active infection or inflammation. See below for more details on pyospermia.
3) Improper semen collection. Most of the prostate fluid is in the first third of the ejaculate, while the seminal vesicle fluid is generally in the last part of the specimen. If part of the collection is missed, this can have an impact on the coagulation and liquefaction of the specimen, depending on which part was lost.
Persistent high sperm viscosity or elevated liquefaction times are rare in the absence of dehydration or infection. Repeat semen analysis testing with the patient well-hydrated and collecting the full specimen usually results in the normalization of the liquefaction/viscosity. However, in those circumstances in which the abnormality persists (as shown by testing at a fertility-specific lab), this could decrease the fertility potential of the couple by inhibiting release of sperm from the coagulated ejaculate. This problem can generally be overcome with low-tech treatments from the female side like sperm washing combined with intrauterine insemination/IUI (see "Female Fertility Treatments" section). If questions arise concerning the fertility impact of abnormal semen liquefaction, post-coital testing can be done to see if sperm are efficiently making it into the cervical canal (see “Post-Coital Testing” in "Uncommonly Used Sperm Tests" section of this website).
Antisperm Antibodies on Semen Analysis Testing
In normal circumstances, sperm are made in what is called an “immunologically privileged” area, in which they are relatively shielded from the body’s immune system. When these barriers of protection are breached, then the immune system can form antibodies against its own sperm. Some of these antisperm antibodies (ASAs) just float around in the bloodstream, while others can attach to the sperm itself. Usually ASAs are not clinically significant, and most men with them have no fertility problems. However, in a minority of men, these antibodies can cause problems with conceiving a child. In men who test positive for ASAs, there is currently no way to predict whether the antibodies are going to definitely cause fertility problems, other than their impact on standard semen parameters (see below).
Common causes of ASA formation include:
1) Significant trauma to the scrotal region
2) Genital duct infections or inflammation
3) Surgery in the scrotal or genital duct region (including vasectomy)
4) Genital tract obstruction
5) Idiopathic (no causative event known)
DIAGNOSIS OF ANTISPERM ANTIBODIES
The presence of antisperm antibodies (ASA) are usually suspected when a semen analysis shows a very low motility and/or low grade of motility along with agglutination. Agglutination is when the sperm are stuck together in larger bunches (called “clumping”). Good fertility-specific labs will comment on the presence of the sperm agglutination in a semen sample. Further testing for anti-sperm antibodies can then be done. There are two types of testing for ASA that can be performed: “Indirect” and “Direct” testing.
INDIRECT ASA TESTING
In indirect testing, a blood test looks for the presence of circulating antibodies in the bloodstream. It’s important to note that antibodies circulating in the bloodstream have not been found to have a significant impact on male fertility. I mention this type of testing here because it can be useful for men who have the combination of azoospermia, normal FSH, normal testicular volume, and no reason to have an obstruction (such as previous hernia repair, vasectomy, etc.). In these men, it is often unclear whether there is a sperm blockage problem or, alternatively, maturation arrest (see “Azoospermia” section for more information on maturation arrest).
About 70 percent of men whose azoospermia is caused by an obstruction will be positive for anti-sperm antibodies on indirect ASA testing of the blood, whereas men with nonobstructive azoospermia should not have any anti-sperm antibodies. (However, men with congenital blockages or sperm transport problems will likely not have elevated levels of direct ASA, as opposed to men with an acquired blockage, who usually will.) This information may help to guide treatment options, although without a testicular biopsy it cannot be definitely determined whether the problem is one of production or one of blockage.
DIRECT ASA TESTING
Direct (immunobead) testing, by contrast, looks for antibodies directly attached to the sperm themselves and determines what percentage of sperm have antibodies attached to them. If 20 percent or more of the sperm have antibodies attached to them, that is generally considered significant. The type of antibody present may also be tested for, with immunoglobulin A (IgA) thought to have a more significant impact on fertility than immunoglobulin G (IgG).
ANTISPERM ANTIBODIES AND FERTILITY
Anti-sperm antibodies can impact fertility in one of two ways:
1) Sperm motility
ASAs can decrease motility by causing sperm to clump together. Clumped sperm do not swim well, and so they can have trouble traveling up the fallopian tubes to reach an egg.
2) Egg-Sperm Interactions
The second possibility is that antibodies can attach to the head of the sperm, interfering with the normal sperm-egg interactions that are necessary for egg fertilization.
ANTI-SPERM ANTIBODIES AND VASECTOMY REVERSALS
Most men (70 to 80 percent or more in some studies) who have been tested using the Indirect ASA test following a vasectomy have been found to have anti-sperm antibodies in their bloodstream. It is a common misconception that these anti-sperm antibodies significantly decrease fertility in men who have their vasectomy reversed. In reality, most men who undergo a successful vasectomy reversal have no ASA-related fertility problems afterward. There is even some evidence to suggest that vasectomy reversal may decrease or even eliminate the presence of ASAs. See the section on "Post-Vasectomy Fertility Options" for more information regarding vasectomy reversals.
MANAGEMENT OF ANTISPERM ANTIBODIES
If direct (immunobead) testing shows that ASAs are impacting more than 20 percent of sperm and there is a significant decrease in sperm motility, then there are several potential treatment options. Of note, some clinicians will treat for suspected ASA’s without formal immunobead testing if there is high clinical suspicion (significant clumping of sperm and poor motility).
SPERM WASHING
One is to combine sperm washing with intrauterine insemination (IUI). The washing process can sometimes break up the agglutinated sperm and improve sperm motility. Before proceeding with IUI, a test wash can be done at the time of a semen analysis to see if the washing process will significantly improve motility. Small studies have shown a pregnancy rate of up to 15–30 percent when IUI is combined with sperm washing.
MEDICAL THERAPY
A second approach is via the immune system. Since antibodies against sperm are an immunologic reaction, using corticosteroids to slightly suppress the immune system should have the potential to decrease antibody levels and improve sperm quality. However, studies looking at whether this works have had mixed results. To see if there is any benefit for a particular man, a doctor can prescribe a ten-day course of prednisone (e.g. 10 mg by mouth once per day) prior to a repeat semen analysis. If significant improvements in sperm motility are seen on the new semen analysis, then the man can take prednisone 10 mg daily for days 1 through 10 of the woman’s menstrual cycle (with day 1 defined as the day of first full menstrual flow). A possible alternative is for the man to take prednisone 20 mg daily for days 1 through 10 of the woman’s menstrual cycle, followed by prednisone 5 mg daily for days 11 through 14 of her cycle (the lower dose serves to taper off from the higher initial dose; higher doses of corticosteroids should never be stopped abruptly).
The steroids can be used for as long as a year, with repeat semen analysis testing every three months to ensure that the man is continuing to respond to the corticosteroids. If longer term steroid regimens are to be used, some physicians recommend taking an anti-ulcer medication such as ranitidine (Zantac), famotidine (Pepcid), or nizatidine (Axid) while on the steroids, to decrease the risk of gastrointestinal side effects.
The use of corticosteroids is not without risks. Fortunately, severe side effects are uncommon, but it is important to be aware of them. Potential side effects include:
1) Aseptic necrosis of the hip and compression fracture of bones (rare, but can necessitate hip replacement or cause permanent
disability)
2) Mood changes
3 )Altered glucose metabolism, possibly indicating diabetes
4) New onset or worsening of peptic ulcer disease
5) Acne
6) Gastrointestinal side effects (diarrhea, bloating, increased gas)
7) Weight gain
8) Facial flushing
9) Impaired wound healing
10) Fluid retention
11) High blood pressure
12) Glaucoma
13) Insomnia
Side effects are generally related to higher dosages and longer durations of treatment. Men with known risk factors, such as elevated blood sugar levels, glaucoma, congestive heart failure, or peptic ulcer disease, should check with their primary care physician before starting these medications.
COMBINED SPERM WASHING AND MEDICAL THERAPY
If a test wash of the sperm does not improve sperm motility, then sometimes combining corticosteroids with sperm washing can help. A test sperm wash following a seven-to-ten-day course of prednisone 10 mg once a day can be performed to see if this results in improved sperm motility. If significant improvements are seen, then the man can take prednisone 10 mg daily for seven to ten days prior to IUI, or prednisone 20 mg by mouth daily for seven to ten days prior to IUI, followed by prednisone 5 mg by mouth daily for four days (to taper off the higher dosage).
IVF/ICSI
If sperm washes and corticosteroids don’t do the trick, then IVF/ICSI is a very effective treatment. Since a single sperm is directly injected into each egg in the laboratory, the sperm do not need to swim or interact normally with the egg, and therefore the impact of any ASAs is irrelevant. See the "Female Fertility Treatments" section for more information on IVF/ICSI.
Uncommonly Used Sperm Tests
POST-COITAL TESTING
Post-coital testing (PCT) involves evaluating the woman’s cervical mucus microscopically for the presence of sperm following intercourse. The test must be performed in the peri-ovulatory period (when the cervical mucus is most receptive to sperm) and approximately two to eight hours after intercourse. A normal test result is defined as more than 10 sperm per high power field (hpf) under the microscope.
PCT is not commonly performed, but it may be useful when there is concern regarding sperm delivery to the cervical mucus. Potential reasons for ordering PCT testing include:
1) Hyperviscous semen
2) Unexplained infertility
3) Low volume ejaculate with normal sperm count
4) Evaluating the fertility potential of men who are not able to produce a specimen either with masturbation or with a special collection condom
5) Concerns about cervical problems
6) Proximal hypospadias when there are concerns that sperm are not being deposited near the cervix
OTHER EVEN MORE RARELY USED FERTILITY TESTS
Other fertility testing methods that are not used much anymore include several tests to evaluate the sperm/egg interactions needed for normal egg fertilization. In the pre-ICSI era, this was important information, as only standard IVF was available, in which about 100,000 sperm were mixed with each egg in a sterile laboratory dish, so the sperm and egg had to interact together normally. These interactions include such necessary steps as capacitation (biochemical changes within the sperm when it enters into the female reproductive tract that make it able to fertilize an egg) and acrosome reaction (when the sperm binds to the zona pellucida surrounding the egg, it initiates the acrosome reaction within the sperm, causing a release of enzymes from the acrosome cap, which allows the sperm to penetrate the egg and initiate fertilization).
The ability to perform intra-cytoplasmic sperm injection (ICSI), in which a single sperm is injected directly into each egg, has made the determination of egg-sperm interactions less important. Poor fertilization rates with standard IVF are now routinely managed with IVF/ICSI, thereby bypassing the need for cumbersome egg/sperm interaction testing. These tests are briefly described for historical purposes only, as they are only rarely used now. These outdated tests include:
ACROSOME REACTION TESTING
The acrosome reaction test was designed to see if the sperm has a normal release of acrosomal enzymes. Used in the past for men with profound abnormalities of sperm heads and in couples with standard IVF failure. Downsides included no absolute cutoffs for normal values and limited lab availability.
SPERM PENETRATION ASSAY
Tests the ability of sperm to penetrate an egg to initiate fertilization. Sperm are mixed in the lab with hamster ova and evaluated for their ability to penetrate the egg.
SPERM ZONA BINDING
Tests the ability of the sperm to attach to the zona pellucida (outer covering of the egg).